|
|
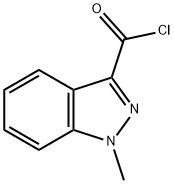
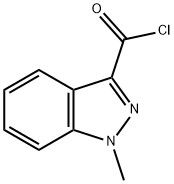
95+ White Powder Granisetron Impurity 13 CAS 106649-02-9
|
Product Details:
Payment & Shipping Terms:
|
| Product Name: | Granisetron Impurity 13 | CAS No: | 106649-02-9 |
|---|---|---|---|
| M.W: | 194.62 | MF: | C9H7ClN2O |
| Appearance: | White Powder | Purity: | 95+ |
| EINECS No: | - | Storage: | 5-25°C |
| Highlight: | CAS 106649-02-9 Granisetron Impurity 13,Granisetron Impurity 13 White Powder,95 Granisetron Impurity 13 |
||
Product Description
White Powder Granisetron Impurity 13 CAS 106649-02-9 95+
Name: Granisetron Impurity 13
CAS NO: 106649-02-9
M.W: 194.62
Appearance: White Powder
Purity: 95+
Synonyms: Granisetron Impurity 13
Application
With regards to the application of Granisetron Impurity 13, there is currently limited information available in the existing knowledge base and literature. In drug development and production, the control and study of impurities are crucial, as they play a significant role in ensuring the quality and safety of drugs. However, specific applications or impacts of a particular impurity, such as Granisetron Impurity 13, often require consultation of the latest scientific research or professional sources.
If you are interested in the application of Granisetron Impurity 13, it is advisable to consult the latest drug development literature, drug quality standards, and relevant research reports. These resources typically provide detailed information on the nature, impact, and role of drug impurities in drug development and quality control.
Furthermore, you may contact professional drug development institutions, pharmaceutical companies, or regulatory agencies for more specific and accurate information. These organizations often possess expertise and experience in addressing questions related to drug impurities and can provide authoritative answers and guidance.
Package
![]()
![]()
![]()
![]()
Transportation
Small package(1g, 25g, 1Kg, 25Kg) can be shipped by Express. (DHL, FedEx, EMS, etc.)
Large package(100kg and more than100Kg) can be transported by Air or Sea.
All transportation is in accordance with the customer's needs.
Company Profile
![]()
![]()
![]()
![]()
![]()
![]()
Contact Person: admin
-
98+ White Powder Impurities(Standards) 6-Chloro-N-Methylpyridine-2-Carboxamide CAS 845306-04-9
-
98+ White Powder TICAGRELOR CAS 1129683-88-0 C14H14N4O3
-
98+ White Powder Impurities(Standards) Sorafenib Impurity 16 CAS 2206827-12-3
-
98+ White Powder Sorafenib Related Compound 8 NO. 1431697-81-2 CAS
-
98+ White Powder Sorafenib Impurity 6 CAS 1285533-84-7
-
Pure 98+ White Powder Sorafenib Impurity 3 CAS NO. 284670-98-0 C27H24N6O5