|
|
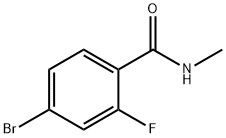
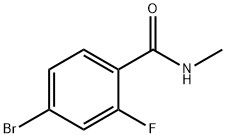
95+ White Powder EnzalutamideImpurity14 CAS NO. 749927-69-3
|
Product Details:
Payment & Shipping Terms:
|
| Product Name: | EnzalutamideImpurity14 | CAS No: | 749927-69-3 |
|---|---|---|---|
| M.W: | 232.05 | MF: | C8H7BrFNO |
| Appearance: | White Powder | Purity: | 95+ |
| EINECS No: | - | Storage: | 5-25°C |
| Highlight: | Enzalutamide Impurity 14 powder,White Enzalutamide Impurity standard,CAS 749927-69-3 impurity |
||
Product Description
White Powder EnzalutamideImpurity14 CAS NO. 749927-69-3 Purity 95+
Name: EnzalutamideImpurity14
CAS NO: 749927-69-3
M.W: 232.05
Appearance: White Powder
Purity: 95+
Synonyms: EnzalutamideEPImpurityB;EnzalutamideImpurity14;N-METHYL4-BROMO-2-FLUORO;
Application
Drug production: During the production of Enzalutamide, manufacturers need to closely monitor potential impurities, including Enzalutamide Impurity 14. Through strict quality control measures, the purity and safety of the final product can be ensured.
Quality control: Pharmaceutical companies must follow strict quality standards, including those set by the International Conference on Harmonization (ICH). These standards require detailed analysis and limits on impurities in drugs to ensure patient safety.
Regulatory compliance: When submitting new drug applications or updating existing drug information to regulatory agencies (such as the US Food and Drug Administration FDA or the European Medicines Agency EMA), manufacturers must provide detailed data on drug impurities, including Enzalutamide Impurity 14.
Research and development: When developing new synthetic routes or optimizing existing drug production processes, researchers need to evaluate the impact of different impurities on drug performance. This research helps to better understand how to control and minimize impurity formation.
Safety assessment: Before a drug enters the market, extensive safety assessments are conducted, including evaluating the potential impact of impurities such as Enzalutamide Impurity 14 on patient health.
Package
![]()
![]()
![]()
![]()
Transportation
Small package(1g, 25g, 1Kg, 25Kg) can be shipped by Express. (DHL, FedEx, EMS, etc.)
Large package(100kg and more than100Kg) can be transported by Air or Sea.
All transportation is in accordance with the customer's needs.
Company Profile
![]()
![]()
![]()
![]()
![]()
![]()
Contact Person: admin
-
98+ White Powder Impurities(Standards) 6-Chloro-N-Methylpyridine-2-Carboxamide CAS 845306-04-9
-
98+ White Powder TICAGRELOR CAS 1129683-88-0 C14H14N4O3
-
98+ White Powder Impurities(Standards) Sorafenib Impurity 16 CAS 2206827-12-3
-
98+ White Powder Sorafenib Related Compound 8 NO. 1431697-81-2 CAS
-
98+ White Powder Sorafenib Impurity 6 CAS 1285533-84-7
-
Pure 98+ White Powder Sorafenib Impurity 3 CAS NO. 284670-98-0 C27H24N6O5