|
|
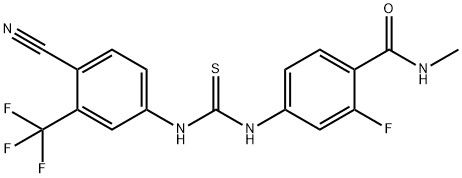
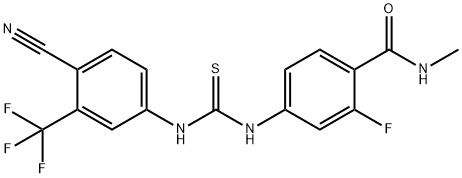
95+ White Powder Enzalutamide Impurity C CAS NO. 1798807-39-2
|
Product Details:
Payment & Shipping Terms:
|
| Product Name: | Enzalutamide Impurity C | CAS No: | 1798807-39-2 |
|---|---|---|---|
| M.W: | 396.36 | MF: | C17H12F4N4OS |
| Appearance: | White Powder | Purity: | 95+ |
| EINECS No: | - | Storage: | 5-25°C |
| Highlight: | Enzalutamide Impurity C powder,White Enzalutamide Impurity C,Enzalutamide Impurity C CAS 1798807-39-2 |
||
Product Description
White Powder Enzalutamide Impurity C CAS NO. 1798807-39-2 Purity 95+
Name: Enzalutamide Impurity C
CAS NO: 1798807-39-2
M.W: 396.36
Appearance: White Powder
Purity: 95+
Synonyms: Benzamide, 4-[[[[4-cyano-3-(trifluoromethyl)phenyl]amino]thioxomethyl]amino]-2-fluoro-N-methyl-;Enzalutamide Impurity C
Application
Enzalutamide Impurity C is a potential impurity found in the production of Enzalutamide. As with other impurities, it is a substance that needs to be closely monitored and controlled during the manufacturing process to ensure the purity and safety of the final drug product.
However, it is important to note that impurities like Enzalutamide Impurity C are not typically used in their own right for any industry applications. Instead, they are considered undesirable components that need to be minimized or removed to ensure the quality and safety of the drug.
The main focus when it comes to Enzalutamide Impurity C is on how to effectively control its formation during the production of Enzalutamide. This involves following strict quality control measures to identify and limit the levels of impurities in the final drug product.
Package
![]()
![]()
![]()
![]()
Transportation
Small package(1g, 25g, 1Kg, 25Kg) can be shipped by Express. (DHL, FedEx, EMS, etc.)
Large package(100kg and more than100Kg) can be transported by Air or Sea.
All transportation is in accordance with the customer's needs.
Company Profile
![]()
![]()
![]()
![]()
![]()
![]()
Contact Person: admin
-
98+ White Powder Impurities(Standards) 6-Chloro-N-Methylpyridine-2-Carboxamide CAS 845306-04-9
-
98+ White Powder TICAGRELOR CAS 1129683-88-0 C14H14N4O3
-
98+ White Powder Impurities(Standards) Sorafenib Impurity 16 CAS 2206827-12-3
-
98+ White Powder Sorafenib Related Compound 8 NO. 1431697-81-2 CAS
-
98+ White Powder Sorafenib Impurity 6 CAS 1285533-84-7
-
Pure 98+ White Powder Sorafenib Impurity 3 CAS NO. 284670-98-0 C27H24N6O5