|
|
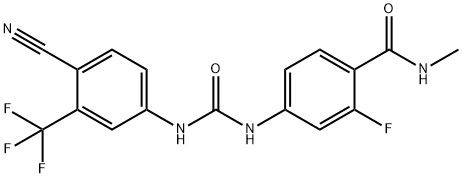
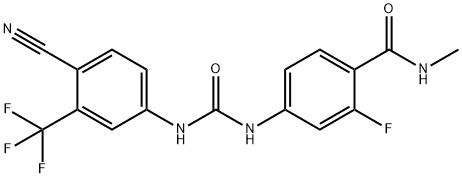
95+ White Powder Enzalutamide Impurity 43 CAS NO. 1351185-73-3
|
Product Details:
Payment & Shipping Terms:
|
| Product Name: | Enzalutamide Impurity 43 | CAS No: | 1351185-73-3 |
|---|---|---|---|
| M.W: | 380.3 | MF: | C17H12F4N4O2 |
| Appearance: | White Powder | Purity: | 95+ |
| EINECS No: | - | Storage: | 5-25°C |
| Highlight: | Enzalutamide Impurity 43 powder,White Enzalutamide Impurity standard,CAS 1351185-73-3 impurity |
||
Product Description
White Powder Enzalutamide Impurity 43 CAS NO. 1351185-73-3 Purity 95+
Name: Enzalutamide Impurity 43
CAS NO: 1351185-73-3
M.W: 380.3
Appearance: White Powder
Purity: 95+
Synonyms: Benzamide, 4-[[[[4-cyano-3-(trifluoromethyl)phenyl]amino]carbonyl]amino]-2-fluoro-N-methyl-;Enzalutamide Impurity 43
Application
Impurities are any substances present in a drug other than the intended active ingredient. They can arise from unreacted starting materials, byproducts, degradation products, or substances introduced during the manufacturing process. In drug production, it is crucial to strictly control impurities to ensure the safety and efficacy of the medication.
The study and control of impurities play a vital role in drug research, development, and production. Impurities can potentially affect the stability, safety, and effectiveness of drugs, necessitating thorough investigation and analysis to guarantee the quality and safety of the final product.
As an impurity related to Enzalutamide, Enzalutamide Impurity 43 may impact the drug's quality and safety profile. Therefore, its detection and control are essential during the production and quality control processes of Enzalutamide.
Package
![]()
![]()
![]()
![]()
Transportation
Small package(1g, 25g, 1Kg, 25Kg) can be shipped by Express. (DHL, FedEx, EMS, etc.)
Large package(100kg and more than100Kg) can be transported by Air or Sea.
All transportation is in accordance with the customer's needs.
Company Profile
![]()
![]()
![]()
![]()
![]()
![]()
Contact Person: admin
-
98+ White Powder Impurities(Standards) 6-Chloro-N-Methylpyridine-2-Carboxamide CAS 845306-04-9
-
98+ White Powder TICAGRELOR CAS 1129683-88-0 C14H14N4O3
-
98+ White Powder Impurities(Standards) Sorafenib Impurity 16 CAS 2206827-12-3
-
98+ White Powder Sorafenib Related Compound 8 NO. 1431697-81-2 CAS
-
98+ White Powder Sorafenib Impurity 6 CAS 1285533-84-7
-
Pure 98+ White Powder Sorafenib Impurity 3 CAS NO. 284670-98-0 C27H24N6O5