|
|
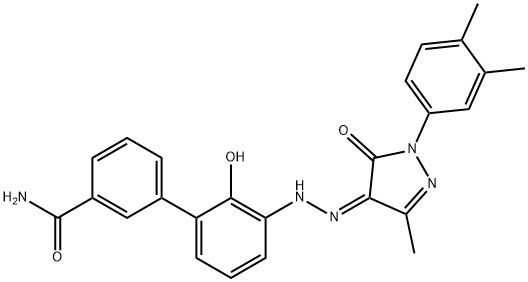
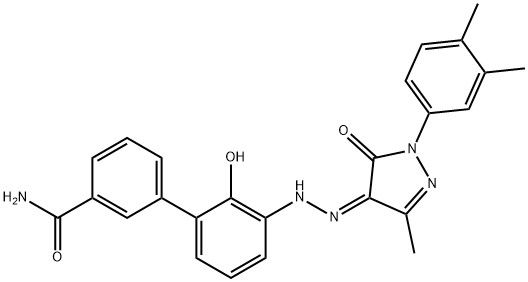
95+ White Powder Eltrombopag Impurity 1 CAS 1246929-02-1
|
Product Details:
Payment & Shipping Terms:
|
| Product Name: | Eltrombopag Impurity 1 | CAS No: | 1246929-02-1 |
|---|---|---|---|
| M.W: | 441.48 | MF: | C25H23N5O3 |
| Appearance: | White Powder | Purity: | 95+ |
| EINECS No: | - | Storage: | 5-25°C |
| Highlight: | Eltrombopag Impurity 1 standard,White powder Eltrombopag Impurity,CAS 1246929-02-1 impurity |
||
Product Description
White Powder Eltrombopag Impurity 1 CAS 1246929-02-1 Purity 95+
Name: Eltrombopag Impurity 1
CAS NO: 1246929-02-1
M.W: 441.48
Appearance: White Powder
Purity: 95+
Synonyms: Eltrombopag Impurity 1
Application
Eltrombopag Impurity 1 refers to a specific impurity that may be present in eltrombopag products. Eltrombopag is a drug used to treat thrombocytopenia, a condition where the body has a low platelet count. Impurities in drugs can potentially affect their safety, efficacy, and stability.
Eltrombopag Impurity 1 is an unwanted by-product that may form during the manufacturing process or as a result of degradation over time. The presence of this impurity may affect the quality of the final drug product and could potentially lead to adverse effects in patients.
To ensure the safety and efficacy of eltrombopag products, manufacturers are required to carefully control the levels of impurities, including Eltrombopag Impurity 1. Stringent quality control measures are implemented to monitor and limit the amount of this impurity in the final drug product.
If Eltrombopag Impurity 1 is detected in a drug product, it may require additional testing and evaluation to assess its impact on the safety and effectivenesss of the drug. In some cases, the presence of unacceptable levels of impurities may lead to the recall of affected batches or the withdrawal of the drug from the market.
Package
![]()
![]()
![]()
![]()
Transportation
Small package(1g, 25g, 1Kg, 25Kg) can be shipped by Express. (DHL, FedEx, EMS, etc.)
Large package(100kg and more than100Kg) can be transported by Air or Sea.
All transportation is in accordance with the customer's needs.
Company Profile
![]()
![]()
![]()
![]()
![]()
![]()
Contact Person: admin
-
98+ White Powder Impurities(Standards) 6-Chloro-N-Methylpyridine-2-Carboxamide CAS 845306-04-9
-
98+ White Powder TICAGRELOR CAS 1129683-88-0 C14H14N4O3
-
98+ White Powder Impurities(Standards) Sorafenib Impurity 16 CAS 2206827-12-3
-
98+ White Powder Sorafenib Related Compound 8 NO. 1431697-81-2 CAS
-
98+ White Powder Sorafenib Impurity 6 CAS 1285533-84-7
-
Pure 98+ White Powder Sorafenib Impurity 3 CAS NO. 284670-98-0 C27H24N6O5