|
|
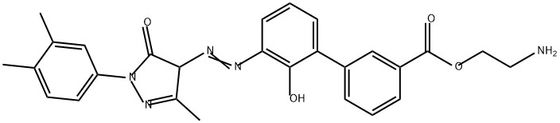
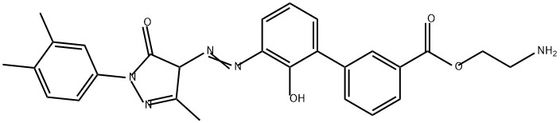
95+ White Powder Etrapopa Ethanolamine impurity 8 CAS 2587607-84-7
|
Product Details:
Payment & Shipping Terms:
|
| Product Name: | Etrapopa Ethanolamine Impurity 8 | CAS No: | 2587607-84-7 |
|---|---|---|---|
| M.W: | 485.53 | MF: | C27H27N5O4 |
| Appearance: | White Powder | Purity: | 95+ |
| EINECS No: | - | Storage: | 5-25°C |
| Highlight: | White Powder Etrapopa Ethanolamine impurity,CAS 2587607-84-7 impurity standard,Etrapopa Ethanolamine impurity with warranty |
||
Product Description
White Powder Etrapopa Ethanolamine impurity 8 CAS 2587607-84-7 Purity 95+
Name: Etrapopa Ethanolamine impurity 8
CAS NO: 2587607-84-7
M.W: 485.53
Appearance: White Powder
Purity: 95+
Synonyms: Etrapopa Ethanolamine impurity 8
Application
EtrapopaEthanolamine impurity 8, also known as Eltrombopag Ethanolamine impurity 8 with a CAS number of 2587607-84-7, is an impurity that may arise during the manufacturing process of certain pharmaceutical drugs. However, there are currently no known applications for this impurity as it is typically not used for any therapeutic or practical purposes.
In pharmaceutical research and development, as well as manufacturing, strict control and monitoring of such impurities are crucial. The presence of impurities can potentially affect the quality and safety of drugs, increasing the risk of adverse reactions or compromising their therapeutic effectiveness. Therefore, pharmaceutical companies employ various measures to minimize the occurrence of these impurities.
The specific chemical properties of EtrapopaEthanolamine impurity 8, including its English name and possible molecular formula, are primarily used for quality control and monitoring during drug development and production, rather than for any specific applications.
Package
![]()
![]()
![]()
![]()
Transportation
Small package(1g, 25g, 1Kg, 25Kg) can be shipped by Express. (DHL, FedEx, EMS, etc.)
Large package(100kg and more than100Kg) can be transported by Air or Sea.
All transportation is in accordance with the customer's needs.
Company Profile
![]()
![]()
![]()
![]()
![]()
![]()
Contact Person: admin
-
98+ White Powder Impurities(Standards) 6-Chloro-N-Methylpyridine-2-Carboxamide CAS 845306-04-9
-
98+ White Powder TICAGRELOR CAS 1129683-88-0 C14H14N4O3
-
98+ White Powder Impurities(Standards) Sorafenib Impurity 16 CAS 2206827-12-3
-
98+ White Powder Sorafenib Related Compound 8 NO. 1431697-81-2 CAS
-
98+ White Powder Sorafenib Impurity 6 CAS 1285533-84-7
-
Pure 98+ White Powder Sorafenib Impurity 3 CAS NO. 284670-98-0 C27H24N6O5