|
|
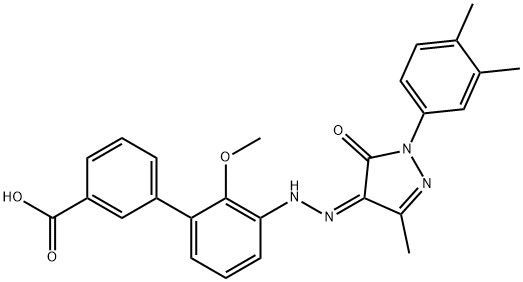
95+ White Powder Etrapopa Ethanolamine impurity CAS 1437383-35-1
|
Product Details:
Payment & Shipping Terms:
|
| Product Name: | Etrapopa Ethanolamine Impurity | CAS No: | 1437383-35-1 |
|---|---|---|---|
| M.W: | 456.49 | MF: | C26H24N4O4 |
| Appearance: | White Powder | Purity: | 95+ |
| EINECS No: | - | Storage: | 5-25°C |
| Highlight: | White Powder Etrapopa Ethanolamine impurity,Etrapopa Ethanolamine CAS 1437383-35-1,Pharmaceutical impurity standard with warranty |
||
Product Description
White Powder Etrapopa Ethanolamine impurity CAS 1437383-35-1 Purity 95+
Name: Etrapopa Ethanolamine impurity
CAS NO: 1437383-35-1
M.W: 456.49
Appearance: White Powder
Purity: 95+
Synonyms: Etrapopa Ethanolamine impurity
Application
EtrapopaEthanolamine impurity, also referred to as eltrombopag ethanolamine impurity, is a potential impurity that may arise during the synthesis or manufacturing of eltrombopag, a drug used to treat thrombocytopenia and other blood disorders. This impurity does not have direct therapeutic applications but rather serves as an indicator for quality control and safety evaluation of the drug.
In the pharmaceutical industry, the presence of impurities like EtrapopaEthanolamine impurity is carefully monitored and controlled to ensure the safety and efficacy of the final drug product. The study and analysis of this impurity are crucial for understanding its origin, quantity, and potential effects on the stability, purity, and safety of eltrombopag.
The primary application of EtrapopaEthanolamine impurity lies in quality control and assurance during drug development and production. Manufacturers employ rigorous analytical methods to detect and quantify this impurity, allowing them to adjust production processes, optimize manufacturing techniques, and ensure that the final drug product meets the required standards for safety and efficacy.
Additionally, the characterization of EtrapopaEthanolamine impurity may contribute to pharmacokinetic and metabolism studies. By understanding how this impurity behaves in the body, researchers can gain insights into the metabolic pathways of eltrombopag and the formation of its metabolites. This information can guide further optimization of the drug and its safety profile.
Package
![]()
![]()
![]()
![]()
Transportation
Small package(1g, 25g, 1Kg, 25Kg) can be shipped by Express. (DHL, FedEx, EMS, etc.)
Large package(100kg and more than100Kg) can be transported by Air or Sea.
All transportation is in accordance with the customer's needs.
Company Profile
![]()
![]()
![]()
![]()
![]()
![]()
Contact Person: admin
-
98+ White Powder Impurities(Standards) 6-Chloro-N-Methylpyridine-2-Carboxamide CAS 845306-04-9
-
98+ White Powder TICAGRELOR CAS 1129683-88-0 C14H14N4O3
-
98+ White Powder Impurities(Standards) Sorafenib Impurity 16 CAS 2206827-12-3
-
98+ White Powder Sorafenib Related Compound 8 NO. 1431697-81-2 CAS
-
98+ White Powder Sorafenib Impurity 6 CAS 1285533-84-7
-
Pure 98+ White Powder Sorafenib Impurity 3 CAS NO. 284670-98-0 C27H24N6O5