|
|
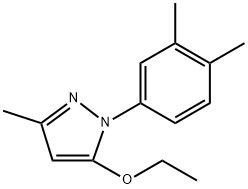
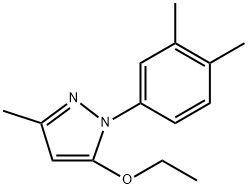
95+ White Powder Etrapopa Ethanolamine impurity 17 CAS 2500321-37-7
|
Product Details:
Payment & Shipping Terms:
|
| Product Name: | Etrapopa Ethanolamine Impurity 17 | CAS No: | 2500321-37-7 |
|---|---|---|---|
| M.W: | 230.31 | MF: | C14H18N2O |
| Appearance: | White Powder | Purity: | 95+ |
| EINECS No: | - | Storage: | 5-25°C |
| Highlight: | Ethanolamine impurity 17 CAS 2500321-37-7,White Powder Etrapopa Ethanolamine impurity,Pharmaceutical impurity standard with CAS number |
||
Product Description
White Powder Etrapopa Ethanolamine impurity 17 CAS 2500321-37-7 Purity 95+
Name: Etrapopa Ethanolamine impurity 17
CAS NO: 2500321-37-7
M.W: 230.31
Appearance: White Powder
Purity: 95+
Synonyms: Etrapopa Ethanolamine impurity 17
Application
Drug Development: In the process of drug development, E17 may emerge as a by-product or intermediate in the synthetic pathway. Understanding and controlling this impurity is crucial to ensuring the safety and efficacy of the final drug product.
Quality Control: In the pharmaceutical industry, quality control is paramount. The presence and quantity of E17 may need to be monitored and controlled to ensure that the drug meets quality standards. This can be achieved through analytical methods and quality control procedures.
Environmental Assessment: If E17 is released into the environment during production, it may require environmental risk assessment. This typically involves studying its environmental persistence, biodegradability, and potential ecological toxicity.
Toxicology Studies: In drug development, understanding the potential toxicity of impurities is essential. E17 may undergo toxicology studies to assess its potential risks to humans and animals.
Package
![]()
![]()
![]()
![]()
Transportation
Small package(1g, 25g, 1Kg, 25Kg) can be shipped by Express. (DHL, FedEx, EMS, etc.)
Large package(100kg and more than100Kg) can be transported by Air or Sea.
All transportation is in accordance with the customer's needs.
Company Profile
![]()
![]()
![]()
![]()
![]()
![]()
Contact Person: admin
-
98+ White Powder Impurities(Standards) 6-Chloro-N-Methylpyridine-2-Carboxamide CAS 845306-04-9
-
98+ White Powder TICAGRELOR CAS 1129683-88-0 C14H14N4O3
-
98+ White Powder Impurities(Standards) Sorafenib Impurity 16 CAS 2206827-12-3
-
98+ White Powder Sorafenib Related Compound 8 NO. 1431697-81-2 CAS
-
98+ White Powder Sorafenib Impurity 6 CAS 1285533-84-7
-
Pure 98+ White Powder Sorafenib Impurity 3 CAS NO. 284670-98-0 C27H24N6O5