|
|
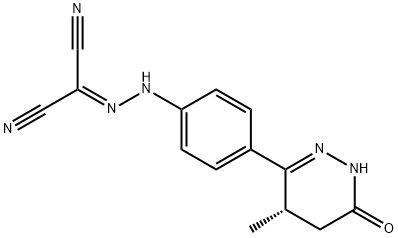
95+ White Powder Levosimendan Impurity 14 CAS 144238-75-5
|
Product Details:
Payment & Shipping Terms:
|
| Product Name: | Levosimendan Impurity 14 | CAS No: | 144238-75-5 |
|---|---|---|---|
| M.W: | 280.28 | MF: | C14H12N6O |
| Appearance: | White Powder | Purity: | 95+ |
| EINECS No: | - | Storage: | 5-25°C |
| Highlight: | Levosimendan Impurity 14 powder,White Levosimendan Impurity standard,CAS 144238-75-5 pharmaceutical impurity |
||
Product Description
White Powder Levosimendan Impurity 14 CAS 144238-75-5 Purity 95+
Name: Levosimendan Impurity 14
CAS NO: 144238-75-5
M.W: 280.28
Appearance: White Powder
Purity: 95+
Synonyms: Levosimendan Impurity 14
Application
Quality Control: Levosimendan Impurity 14 is analyzed quantitatively and qualitatively to ensure its levels are below acceptable limits, thereby guaranteeing the purity and quality of the drug. This analysis is crucial during the manufacturing process to monitor and control impurity levels.
Safety Evaluation: Safety assessments of Levosimendan Impurity 14 involve toxicological and pharmacological studies to understand its potential adverse effects on humans. This evaluation ensures the safety of the drug during clinical use.
Process Optimization: Studying Levosimendan Impurity 14 can provide insights into its formation and origin during the manufacturing process. This knowledge can guide the optimization of production processes, aiming to minimize its formation and enhance the stability and purity of the final drug product.
Regulatory Compliance: Complying with pharmaceutical regulations often requires detailed characterization of impurities. Understanding the nature and levels of Levosimendan Impurity 14 helps manufacturers to ensure their products meet regulatory standards and avoid compliance issues.
Package
![]()
![]()
![]()
![]()
Transportation
Small package(1g, 25g, 1Kg, 25Kg) can be shipped by Express. (DHL, FedEx, EMS, etc.)
Large package(100kg and more than100Kg) can be transported by Air or Sea.
All transportation is in accordance with the customer's needs.
Company Profile
![]()
![]()
![]()
![]()
![]()
![]()
Contact Person: admin
-
98+ White Powder Impurities(Standards) 6-Chloro-N-Methylpyridine-2-Carboxamide CAS 845306-04-9
-
98+ White Powder TICAGRELOR CAS 1129683-88-0 C14H14N4O3
-
98+ White Powder Impurities(Standards) Sorafenib Impurity 16 CAS 2206827-12-3
-
98+ White Powder Sorafenib Related Compound 8 NO. 1431697-81-2 CAS
-
98+ White Powder Sorafenib Impurity 6 CAS 1285533-84-7
-
Pure 98+ White Powder Sorafenib Impurity 3 CAS NO. 284670-98-0 C27H24N6O5