|
|
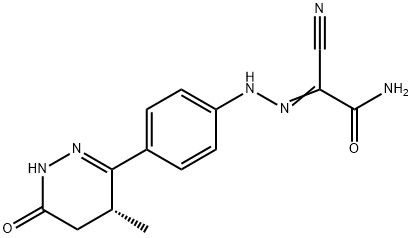
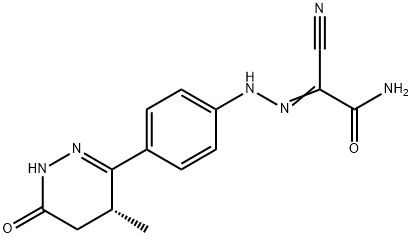
95+ White Powder Levosimendan Impurity 8 CAS 274263-65-9
|
Product Details:
Payment & Shipping Terms:
|
| Product Name: | Levosimendan Impurity 8 | CAS No: | 274263-65-9 |
|---|---|---|---|
| M.W: | 298.3 | MF: | C14H14N6O2 |
| Appearance: | White Powder | Purity: | 95+ |
| EINECS No: | - | Storage: | 5-25°C |
| Highlight: | Levosimendan Impurity 8 powder,White Levosimendan standard,CAS 274263-65-9 impurity |
||
Product Description
White Powder Levosimendan Impurity 8 CAS 274263-65-9 Purity 95+
Name: Levosimendan Impurity 8
CAS NO: 274263-65-9
M.W: 298.3
Appearance: White Powder
Purity: 95+
Synonyms: Levosimendan Impurity 8
Application
Quality Control: Monitoring and controlling the level of Levosimendan Impurity 8 is crucial in ensuring the quality and safety of the final Levosimendan product. Manufacturers need to ensure that the impurity levels meet established quality standards.
Stability Studies: By studying the formation and stability of Levosimendan Impurity 8 under various conditions, scientists can gain insights into the degradation pathways and rates of Levosimendan, thereby predicting its stability during storage and use.
Drug Development: Researching Levosimendan Impurity 8 can aid in optimizing synthetic routes, reducing impurity formation, and improving the purity of the drug during the drug development phase. This not only enhances production efficiency but also helps to reduce production costs.
Safety Assessment: While Levosimendan Impurity 8 itself may not have therapeutic activity, its presence may pose potential risks to human health. Therefore, a thorough safety assessment of the impurity is an essential part of the drug development process.
Regulatory Compliance: Manufacturers are required to demonstrate the safety and efficacy of their products to meet the requirements of drug regulatory agencies. Extensive research on Levosimendan Impurity 8 is an important component of fulfilling these regulatory requirements.
Package
![]()
![]()
![]()
![]()
Transportation
Small package(1g, 25g, 1Kg, 25Kg) can be shipped by Express. (DHL, FedEx, EMS, etc.)
Large package(100kg and more than100Kg) can be transported by Air or Sea.
All transportation is in accordance with the customer's needs.
Company Profile
![]()
![]()
![]()
![]()
![]()
![]()
Contact Person: admin
-
98+ White Powder Impurities(Standards) 6-Chloro-N-Methylpyridine-2-Carboxamide CAS 845306-04-9
-
98+ White Powder TICAGRELOR CAS 1129683-88-0 C14H14N4O3
-
98+ White Powder Impurities(Standards) Sorafenib Impurity 16 CAS 2206827-12-3
-
98+ White Powder Sorafenib Related Compound 8 NO. 1431697-81-2 CAS
-
98+ White Powder Sorafenib Impurity 6 CAS 1285533-84-7
-
Pure 98+ White Powder Sorafenib Impurity 3 CAS NO. 284670-98-0 C27H24N6O5