|
|
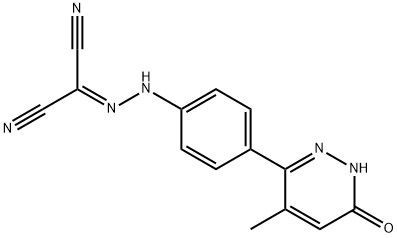
95+ White Powder Levosimendan Impurity 25 CAS 131741-36-1
|
Product Details:
Payment & Shipping Terms:
|
| Product Name: | Levosimendan Impurity 25 | CAS No: | 131741-36-1 |
|---|---|---|---|
| M.W: | 278.27 | MF: | C14H10N6O |
| Appearance: | White Powder | Purity: | 95+ |
| EINECS No: | - | Storage: | 5-25°C |
| Highlight: | Levosimendan impurity 25 CAS 131741-36-1,White powder Levosimendan impurity,Pharmaceutical impurity standard Levosimendan |
||
Product Description
White Powder Levosimendan Impurity 25 CAS 131741-36-1 95+
Name: Levosimendan Impurity 25
CAS NO: 131741-36-1
M.W: 278.27
Appearance: White Powder
Purity: 95+
Synonyms: Levosimendan Impurity 25
Application
Drug Discovery and Development: During the drug discovery and development phase, Levosimendan Impurity 25 is studied and controlled as a key impurity. The analysis and control of impurities in drugs are crucial in ensuring their safety, efficacy, and stability.
Drug Manufacturing: In the manufacturing phase, Levosimendan Impurity 25 may be used to monitor and control the quality of drug products. Pharmaceutical companies need to ensure that the impurity levels in their drugs meet regulatory standards to maintain product safety and effectiveness.
Quality Assurance and Control: In quality control, Levosimendan Impurity 25 can serve as a reference material or control substance for drug analysis, impurity detection, and drug release and stability studies. This helps to ensure that drug products consistently meet quality standards throughout their lifecycle.
Regulatory Compliance: In the realm of drug regulation, pharmaceutical companies need to adhere to various regulations and standards to guarantee the safety and efficacy of their drugs. The study and control of Levosimendan Impurity 25 may be part of these regulations and standards, assisting pharmaceutical companies in fulfilling regulatory requirements.
Package
![]()
![]()
![]()
![]()
Transportation
Small package(1g, 25g, 1Kg, 25Kg) can be shipped by Express. (DHL, FedEx, EMS, etc.)
Large package(100kg and more than100Kg) can be transported by Air or Sea.
All transportation is in accordance with the customer's needs.
Company Profile
![]()
![]()
![]()
![]()
![]()
![]()
Contact Person: admin
-
98+ White Powder Impurities(Standards) 6-Chloro-N-Methylpyridine-2-Carboxamide CAS 845306-04-9
-
98+ White Powder TICAGRELOR CAS 1129683-88-0 C14H14N4O3
-
98+ White Powder Impurities(Standards) Sorafenib Impurity 16 CAS 2206827-12-3
-
98+ White Powder Sorafenib Related Compound 8 NO. 1431697-81-2 CAS
-
98+ White Powder Sorafenib Impurity 6 CAS 1285533-84-7
-
Pure 98+ White Powder Sorafenib Impurity 3 CAS NO. 284670-98-0 C27H24N6O5