|
|
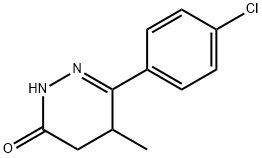
95+ White Powder Levosimendan Impurity CAS 36725-41-4
|
Product Details:
Payment & Shipping Terms:
|
| Product Name: | Levosimendan Impurity | CAS No: | 36725-41-4 |
|---|---|---|---|
| M.W: | 222.67 | MF: | C11H11ClN2O |
| Appearance: | White Powder | Purity: | 95+ |
| EINECS No: | - | Storage: | 5-25°C |
| Highlight: | Levosimendan impurity white powder,Levosimendan impurity CAS 36725-41-4,Pharmaceutical impurity with warranty |
||
Product Description
White Powder Levosimendan Impurity 35 CAS 36725-41-4 95+
Name: Levosimendan Impurity
CAS NO: 36725-41-4
M.W: 222.67
Appearance: White Powder
Purity: 95+
Synonyms: Levosimendan Impurity
Application
Levosimendan, a positive inotropic agent primarily used to treat heart failure, has impurities associated with its synthesis or production. Typically, impurities like Levosimendan Impurities are not directly used for therapeutic purposes. Instead, they are factors that need to be controlled during drug development and production.
The presence of impurities can potentially affect the stability, safety, and efficacy of a drug. Therefore, in-depth studies and control measures are essential for understanding and managing these impurities. Levosimendan Impurities, for instance, might be studied to understand their chemical properties, reasons for their formation, their concentration in the drug product, and methods to control and minimize their presence.
While Levosimendan Impurities themselves may not have direct applications, their study and control are crucial for ensuring the quality and safety of the final drug product. This is vital for safeguarding the interests of patients.
Package
![]()
![]()
![]()
![]()
Transportation
Small package(1g, 25g, 1Kg, 25Kg) can be shipped by Express. (DHL, FedEx, EMS, etc.)
Large package(100kg and more than100Kg) can be transported by Air or Sea.
All transportation is in accordance with the customer's needs.
Company Profile
![]()
![]()
![]()
![]()
![]()
![]()
Contact Person: admin
-
98+ White Powder Impurities(Standards) 6-Chloro-N-Methylpyridine-2-Carboxamide CAS 845306-04-9
-
98+ White Powder TICAGRELOR CAS 1129683-88-0 C14H14N4O3
-
98+ White Powder Impurities(Standards) Sorafenib Impurity 16 CAS 2206827-12-3
-
98+ White Powder Sorafenib Related Compound 8 NO. 1431697-81-2 CAS
-
98+ White Powder Sorafenib Impurity 6 CAS 1285533-84-7
-
Pure 98+ White Powder Sorafenib Impurity 3 CAS NO. 284670-98-0 C27H24N6O5