|
|
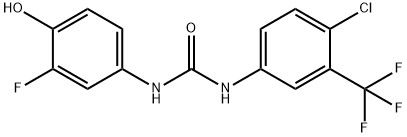
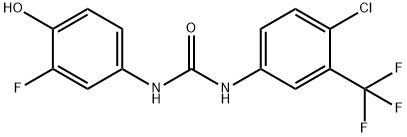
95+ White Powder Regorafenib impurity CAS 2243399-56-4
|
Product Details:
Payment & Shipping Terms:
|
| Product Name: | Regorafenib Impurity 5 | CAS No: | 2243399-56-4 |
|---|---|---|---|
| M.W: | 348.68 | MF: | C14H9ClF4N2O2 |
| Appearance: | White Powder | Purity: | 95+ |
| EINECS No: | - | Storage: | 5-25°C |
| Highlight: | Regorafenib impurity powder,White Regorafenib impurity CAS,Pharmaceutical impurity standard 2243399-56-4 |
||
Product Description
White Powder Regorafenib impurity CAS 2243399-56-4 95+
Name: Regorafenib impurity
CAS NO: 2243399-56-4
M.W: 348.68
Appearance: White Powder
Purity: 95+
Synonyms: Regorafenib impurity
Application
Regorafenib impurity refers to an impurity found in regorafenib, which is an oral multi-kinase inhibitor primarily used to treat certain types of cancer. The production and use of drugs may result in trace amounts of impurities, which may be unavoidable minor components in the manufacturing process or may arise from the degradation of the drug during storage and use.
Specific information about regorafenib impurity, such as its chemical structure, properties, causes of formation, and effects on the human body, requires consultation of professional pharmacological or medicinal chemistry literature or consultation with experts in the relevant field. Controlling impurities is crucial in drug development and production as they can potentially affect the drug's effectiveness and safety. Therefore, pharmaceutical companies typically conduct rigorous impurity analysis and control to ensure the quality and safety of their drugs.
Package
![]()
![]()
![]()
![]()
Transportation
Small package(1g, 25g, 1Kg, 25Kg) can be shipped by Express. (DHL, FedEx, EMS, etc.)
Large package(100kg and more than100Kg) can be transported by Air or Sea.
All transportation is in accordance with the customer's needs.
Company Profile
![]()
![]()
![]()
![]()
![]()
![]()
Contact Person: admin
-
98+ White Powder Impurities(Standards) 6-Chloro-N-Methylpyridine-2-Carboxamide CAS 845306-04-9
-
98+ White Powder TICAGRELOR CAS 1129683-88-0 C14H14N4O3
-
98+ White Powder Impurities(Standards) Sorafenib Impurity 16 CAS 2206827-12-3
-
98+ White Powder Sorafenib Related Compound 8 NO. 1431697-81-2 CAS
-
98+ White Powder Sorafenib Impurity 6 CAS 1285533-84-7
-
Pure 98+ White Powder Sorafenib Impurity 3 CAS NO. 284670-98-0 C27H24N6O5