|
|
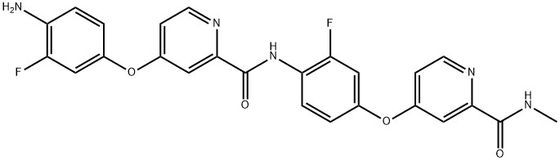
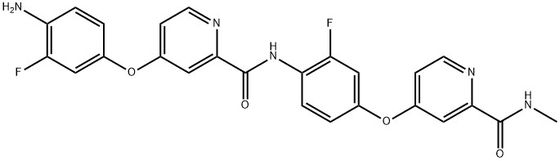
95+ White Powder Regorafenib Impurity 23 CAS 2518234-85-8
|
Product Details:
Payment & Shipping Terms:
|
| Product Name: | Regorafenib Impurity 23 | CAS No: | 2518234-85-8 |
|---|---|---|---|
| M.W: | 491.45 | MF: | C25H19F2N5O4 |
| Appearance: | White Powder | Purity: | 95+ |
| EINECS No: | - | Storage: | 5-25°C |
| Highlight: | Regorafenib Impurity 23 powder,White Regorafenib Impurity standard,CAS 2518234-85-8 Impurity |
||
Product Description
White Powder Regorafenib Impurity 23 CAS 2518234-85-8 95+
Name: Regorafenib Impurity 23
CAS NO: 2518234-85-8
M.W: 491.45
Appearance: White Powder
Purity: 95+
Synonyms: Regorafenib Impurity 23
Application
Regorafenib Impurity 23, as an impurity of regorafenib, does not have a direct therapeutic role in drug applications. However, the in-depth study and analysis of impurities are crucial in drug development, production, and quality control processes.
Firstly, the identification and quantification of Regorafenib Impurity 23 help pharmaceutical companies understand its sources and conditions of formation, enabling them to optimize production processes, reduce impurity generation, and improve drug purity. This is essential for ensuring the safety and effectiveness of the drug.
Secondly, by studying the biological characteristics and potential pharmacological effects of Regorafenib Impurity 23, scientists can gain a better understanding of the mechanism of action of regorafenib in the body, as well as the potential impact of impurities on drug efficacy and safety. This provides more accurate and comprehensive information for the clinical application of the drug.
Furthermore, the monitoring and control of Regorafenib Impurity 23 are integral parts of the drug quality control system. By establishing strict quality standards and detection methods, pharmaceutical companies can ensure that the impurity levels in the drug are within acceptable ranges, thereby safeguarding patient safety during medication.
Package
![]()
![]()
![]()
![]()
Transportation
Small package(1g, 25g, 1Kg, 25Kg) can be shipped by Express. (DHL, FedEx, EMS, etc.)
Large package(100kg and more than100Kg) can be transported by Air or Sea.
All transportation is in accordance with the customer's needs.
Company Profile
![]()
![]()
![]()
![]()
![]()
![]()
Contact Person: admin
-
98+ White Powder Impurities(Standards) 6-Chloro-N-Methylpyridine-2-Carboxamide CAS 845306-04-9
-
98+ White Powder TICAGRELOR CAS 1129683-88-0 C14H14N4O3
-
98+ White Powder Impurities(Standards) Sorafenib Impurity 16 CAS 2206827-12-3
-
98+ White Powder Sorafenib Related Compound 8 NO. 1431697-81-2 CAS
-
98+ White Powder Sorafenib Impurity 6 CAS 1285533-84-7
-
Pure 98+ White Powder Sorafenib Impurity 3 CAS NO. 284670-98-0 C27H24N6O5