|
|
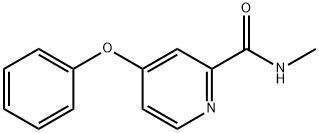
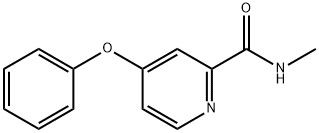
95+ White Powder Sorafenib Impurity 11 CAS 2004659-85-0
|
Product Details:
Payment & Shipping Terms:
|
| Product Name: | Sorafenib Impurity 11 | CAS No: | 2004659-85-0 |
|---|---|---|---|
| M.W: | 228.25 | MF: | C13H12N2O2 |
| Appearance: | White Powder | Purity: | 95+ |
| EINECS No: | - | Storage: | 5-25°C |
| Highlight: | Sorafenib Impurity 11 powder,White Sorafenib Impurity standard,CAS 2004659-85-0 impurity |
||
Product Description
White Powder Sorafenib Impurity 11 CAS 2004659-85-0 95+
Name: Sorafenib Impurity 11
CAS NO: 2004659-85-0
M.W: 228.25
Appearance: White Powder
Purity: 95+
Synonyms: Sorafenib Impurity 11
Application
In drug R&D, a thorough understanding of Sorafenib Impurity 11 can assist scientists in gaining deeper insights into the synthetic pathways and reaction mechanisms of Sorafenib. By analyzing the structure and properties of the impurity, it is possible to optimize the drug's manufacturing process, reducing the formation of impurities and consequently enhancing the purity and stability of the medication. Furthermore, this research may provide insights and inspiration for the development of other similar drugs.
In quality control, Sorafenib Impurity 11 serves as an important marker for drug analysis. Monitoring the quantity and types of impurities in a drug allows for the assessment of its quality and safety, ensuring compliance with relevant regulations and quality standards. Additionally, the detection of Sorafenib Impurity 11 can aid in the identification of abnormalities in the drug production process, enabling timely intervention and resolution to maintain the stability and reliability of drug manufacturing.
Package
![]()
![]()
![]()
![]()
Transportation
Small package(1g, 25g, 1Kg, 25Kg) can be shipped by Express. (DHL, FedEx, EMS, etc.)
Large package(100kg and more than100Kg) can be transported by Air or Sea.
All transportation is in accordance with the customer's needs.
Company Profile
![]()
![]()
![]()
![]()
![]()
![]()
Contact Person: admin
-
98+ White Powder Impurities(Standards) 6-Chloro-N-Methylpyridine-2-Carboxamide CAS 845306-04-9
-
98+ White Powder TICAGRELOR CAS 1129683-88-0 C14H14N4O3
-
98+ White Powder Impurities(Standards) Sorafenib Impurity 16 CAS 2206827-12-3
-
98+ White Powder Sorafenib Related Compound 8 NO. 1431697-81-2 CAS
-
98+ White Powder Sorafenib Impurity 6 CAS 1285533-84-7
-
Pure 98+ White Powder Sorafenib Impurity 3 CAS NO. 284670-98-0 C27H24N6O5