|
|
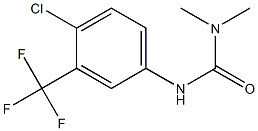
95+ White Powder Regorafenib Impurity 40 CAS 2711-18-4
|
Product Details:
Payment & Shipping Terms:
|
| Product Name: | Regorafenib Impurity 40 | CAS No: | 2711-18-4 |
|---|---|---|---|
| M.W: | 266.6 | MF: | C10H10ClF3N2O |
| Appearance: | White Powder | Purity: | 95+ |
| EINECS No: | - | Storage: | 5-25°C |
| Highlight: | Regorafenib impurity 40 powder,White Regorafenib impurity standard,CAS 2711-18-4 pharmaceutical impurity |
||
Product Description
White Powder Regorafenib Impurity 40 CAS 2711-18-4 95+
Name: Sorafenib Impurity 40
CAS NO: 2711-18-4
M.W: 266.6
Appearance: White Powder
Purity: 95+
Synonyms: Sorafenib Impurity 40
Application
Regarding the application of Sorafenib Impurity 40, there is currently no specific information on its direct industrial or medical use. In the process of drug development and manufacturing, impurities, including Sorafenib Impurity 40, require strict control and monitoring to ensure the purity, stability, and safety of the medication. Therefore, Sorafenib Impurity 40 itself does not possess direct application value.
However, the research and analysis of such impurities hold significant importance in drug research and development (R&D) and quality control. A deep understanding of Sorafenib Impurity 40 can assist scientists in gaining insights into the synthetic pathways and reaction mechanisms of Sorafenib. By studying this impurity, it is possible to optimize the drug manufacturing process, minimizing the formation of impurities and consequently enhancing the purity and quality of the medication. This, in turn, contributes to improving the overall therapeutic effect and safety of the drug, providing better treatment options for patients.
It is crucial to note that due to the specific nature and potential risks associated with Sorafenib Impurity 40 as an impurity, any research or application involving this impurity must undergo rigorous scientific evaluation and approval processes to ensure its safety and effectiveness. Strict adherence to relevant regulations and guidelines is essential to guarantee the quality and safety of the drug.
Package
![]()
![]()
![]()
![]()
Transportation
Small package(1g, 25g, 1Kg, 25Kg) can be shipped by Express. (DHL, FedEx, EMS, etc.)
Large package(100kg and more than100Kg) can be transported by Air or Sea.
All transportation is in accordance with the customer's needs.
Company Profile
![]()
![]()
![]()
![]()
![]()
![]()
Contact Person: admin
-
98+ White Powder Impurities(Standards) 6-Chloro-N-Methylpyridine-2-Carboxamide CAS 845306-04-9
-
98+ White Powder TICAGRELOR CAS 1129683-88-0 C14H14N4O3
-
98+ White Powder Impurities(Standards) Sorafenib Impurity 16 CAS 2206827-12-3
-
98+ White Powder Sorafenib Related Compound 8 NO. 1431697-81-2 CAS
-
98+ White Powder Sorafenib Impurity 6 CAS 1285533-84-7
-
Pure 98+ White Powder Sorafenib Impurity 3 CAS NO. 284670-98-0 C27H24N6O5