|
|
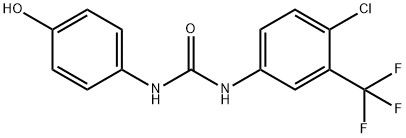
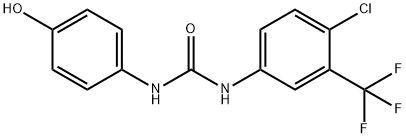
95+ White Powder Sorafenib Impurity 65 CAS 1129683-83-5
|
Product Details:
Payment & Shipping Terms:
|
| Product Name: | Sorafenib Impurity 65 | CAS No: | 1129683-83-5 |
|---|---|---|---|
| M.W: | 330.69 | MF: | C14H10ClF3N2O2 |
| Appearance: | White Powder | Purity: | 95+ |
| EINECS No: | - | Storage: | 5-25°C |
| Highlight: | White Powder Sorafenib Impurity 65,Sorafenib Impurity CAS 1129683-83-5,Pharmaceutical Impurity Standard Sorafenib |
||
Product Description
White Powder Sorafenib Impurity 65 CAS 1129683-83-5 95+
Name: Regorafenib Impurity 65
CAS NO: 1129683-83-5
M.W: 330.69
Appearance: White Powder
Purity: 95+
Synonyms: Regorafenib Impurity 65
Application
Regarding the specific "application" of "Regorafenib Impurity 65," it is not a commonly used term or concept. In drug development and production, the presence of impurities needs to be strictly controlled and managed to ensure the safety, effectiveness, and stability of the medication. Impurities are not intended for any specific application but rather must be reduced to safe levels to prevent negative impacts on the quality and effectiveness of the drug.
Therefore, "Regorafenib Impurity 65" does not represent a term with practical application significance. If it is mentioned in drug research or quality control reports, it is likely used to describe a specific impurity found in the drug Regorafenib, potentially involving information about its nature, origin, detection methods, or control strategies.
For non-professionals, it can be challenging to directly understand or apply detailed information about specific impurities such as "Regorafenib Impurity 65." If you have any questions or concerns regarding impurity control, safety, or effectiveness of Regorafenib or other medications, it is advisable to consult with professional drug researchers, pharmacists, or healthcare professionals. They can provide you with accurate answers and recommendations based on the latest scientific research and clinical practice.
Package
![]()
![]()
![]()
![]()
Transportation
Small package(1g, 25g, 1Kg, 25Kg) can be shipped by Express. (DHL, FedEx, EMS, etc.)
Large package(100kg and more than100Kg) can be transported by Air or Sea.
All transportation is in accordance with the customer's needs.
Company Profile
![]()
![]()
![]()
![]()
![]()
![]()
Contact Person: admin
-
98+ White Powder Impurities(Standards) 6-Chloro-N-Methylpyridine-2-Carboxamide CAS 845306-04-9
-
98+ White Powder TICAGRELOR CAS 1129683-88-0 C14H14N4O3
-
98+ White Powder Impurities(Standards) Sorafenib Impurity 16 CAS 2206827-12-3
-
98+ White Powder Sorafenib Related Compound 8 NO. 1431697-81-2 CAS
-
98+ White Powder Sorafenib Impurity 6 CAS 1285533-84-7
-
Pure 98+ White Powder Sorafenib Impurity 3 CAS NO. 284670-98-0 C27H24N6O5