|
|
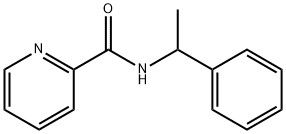
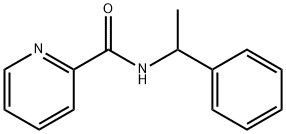
95+ White Powder Regorafenib Impurity 13 CAS 2909-32-2
|
Product Details:
Payment & Shipping Terms:
|
| Product Name: | Regorafenib Impurity 13 | CAS No: | 2909-32-2 |
|---|---|---|---|
| M.W: | 226.27 | MF: | C14H14N2O |
| Appearance: | White Powder | Purity: | 95+ |
| EINECS No: | - | Storage: | 5-25°C |
| Highlight: | Regorafenib Impurity 13 powder,White Regorafenib Impurity standard,CAS 2909-32-2 pharmaceutical impurity |
||
Product Description
White Powder Regorafenib Impurity 13 CAS 2909-32-2 95+
Name: Regorafenib Impurity 13
CAS NO: 2909-32-2
M.W: 226.27
Appearance: White Powder
Purity: 95+
Synonyms: Regorafenib Impurity 13
Application
Regorafenib Impurity 13, as an impurity found in Regorafenib, does not exist for specific applications. In drug development and production, impurities are strictly controlled and managed to ensure the safety, effectiveness, and stability of the medication.
The presence of impurities can potentially have negative impacts on the quality and effectiveness of drugs. Therefore, during drug preparation and quality control, various measures are taken to reduce or eliminate impurities. These include selecting high-quality raw materials, optimizing production processes, using appropriate purification methods, and conducting rigorous quality inspections.
Regarding the specific application of Regorafenib Impurity 13, it does not possess direct applicability. However, for drug researchers and quality control experts, understanding and studying the nature, origin, and potential removal methods of this impurity are crucial. This aids in better controlling the quality of the drug and ensuring its safety and effectiveness in clinical applications.
Package
![]()
![]()
![]()
![]()
Transportation
Small package(1g, 25g, 1Kg, 25Kg) can be shipped by Express. (DHL, FedEx, EMS, etc.)
Large package(100kg and more than100Kg) can be transported by Air or Sea.
All transportation is in accordance with the customer's needs.
Company Profile
![]()
![]()
![]()
![]()
![]()
![]()
Contact Person: admin
-
98+ White Powder Impurities(Standards) 6-Chloro-N-Methylpyridine-2-Carboxamide CAS 845306-04-9
-
98+ White Powder TICAGRELOR CAS 1129683-88-0 C14H14N4O3
-
98+ White Powder Impurities(Standards) Sorafenib Impurity 16 CAS 2206827-12-3
-
98+ White Powder Sorafenib Related Compound 8 NO. 1431697-81-2 CAS
-
98+ White Powder Sorafenib Impurity 6 CAS 1285533-84-7
-
Pure 98+ White Powder Sorafenib Impurity 3 CAS NO. 284670-98-0 C27H24N6O5