|
|
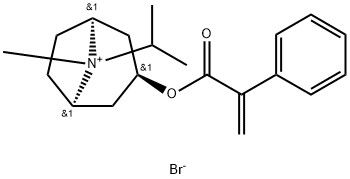
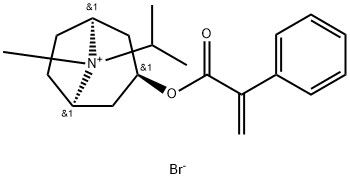
95+ White Powder Ipratropium Bromide EP Impurity F CAS 60018-35-1
|
Product Details:
Payment & Shipping Terms:
|
| Product Name: | Ipratropium Bromide EP Impurity F | CAS No: | 60018-35-1 |
|---|---|---|---|
| M.W: | 394.35 | MF: | C20H28BRNO2 |
| Appearance: | White Powder | Purity: | 95+ |
| EINECS No: | - | Storage: | 5-25°C |
| Highlight: | Ipratropium Bromide EP Impurity F,White Powder Ipratropium Bromide Impurity,CAS 60018-35-1 Impurity Standard |
||
Product Description
White Powder Ipratropium Bromide EP Impurity F CAS 60018-35-1 95+
Name: Ipratropium Bromide EP Impurity F
CAS NO: 60018-35-1
M.W: 394.35
Appearance: White Powder
Purity: 95+
Synonyms: Ipratropium Bromide EP Impurity F
Application
Ipratropium Bromide EP Impurity F, by itself, does not possess independent application value. It is an impurity that arises during the synthesis or production of Ipratropium Bromide. In drug research and development, as well as during production, the presence of impurities requires strict control and management to ensure the purity and safety of the medication.
Ipratropium Bromide is a commonly used drug primarily for the treatment of chronic obstructive pulmonary disease (COPD) and other respiratory conditions. However, any impurities, including Ipratropium Bromide EP Impurity F, can potentially compromise the purity and effectiveness of the drug, and may even pose potential safety risks.
As a result, drug researchers and quality control experts strive to reduce or eliminate such impurities to guarantee the drug's efficacy and patient safety. This may involve optimizing synthesis routes, improving purification techniques, and other steps to minimize the presence of this impurity.
Package
![]()
![]()
![]()
![]()
Transportation
Small package(1g, 25g, 1Kg, 25Kg) can be shipped by Express. (DHL, FedEx, EMS, etc.)
Large package(100kg and more than100Kg) can be transported by Air or Sea.
All transportation is in accordance with the customer's needs.
Company Profile
![]()
![]()
![]()
![]()
![]()
![]()
Contact Person: admin
-
98+ White Powder Impurities(Standards) 6-Chloro-N-Methylpyridine-2-Carboxamide CAS 845306-04-9
-
98+ White Powder TICAGRELOR CAS 1129683-88-0 C14H14N4O3
-
98+ White Powder Impurities(Standards) Sorafenib Impurity 16 CAS 2206827-12-3
-
98+ White Powder Sorafenib Related Compound 8 NO. 1431697-81-2 CAS
-
98+ White Powder Sorafenib Impurity 6 CAS 1285533-84-7
-
Pure 98+ White Powder Sorafenib Impurity 3 CAS NO. 284670-98-0 C27H24N6O5