|
|
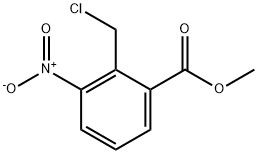
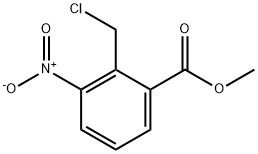
95+ White Powder Lenalidomide Impurity 15 CAS 1218910-61-2
|
Product Details:
Payment & Shipping Terms:
|
| Product Name: | Lenalidomide Impurity 15 | CAS No: | 1218910-61-2 |
|---|---|---|---|
| M.W: | 229.62 | MF: | C9H8ClNO4 |
| Appearance: | White Powder | Purity: | 95+ |
| EINECS No: | - | Storage: | 5-25°C |
| Highlight: | CAS 1218910-61-2 Lenalidomide Impurity 15,95 Lenalidomide Impurity 15,White Powder Lenalidomide Impurity 15 |
||
Product Description
White Powder Lenalidomide Impurity 15 CAS 1218910-61-2 95+
Name: Lenalidomide Impurity 15
CAS NO: 1218910-61-2
M.W: 229.62
Appearance: White Powder
Purity: 95+
Synonyms: Lenalidomide Impurity 15
Application
Regarding the application of Lenalidomide Impurity 15, it is important to note that impurities, in the context of drug development and production, are primarily subject to control and monitoring to ensure the purity and safety of the drug. Therefore, Lenalidomide Impurity 15 itself does not possess any direct clinical application value.
Lenalidomide, an immunomodulatory drug, is primarily used in the treatment of certain hematological malignancies, such as multiple myeloma. However, its impurities, including Lenalidomide Impurity 15, serve more as a parameter that needs to be monitored and controlled during the drug's development and production processes, rather than being a therapeutically active component.
Scientists and pharmaceutical companies closely monitor and control the levels of these impurities to ensure the quality and safety of the final product. High levels of impurities can potentially affect the stability, efficacy, and safety of the drug. Therefore, research on Lenalidomide Impurity 15 is primarily focused on understanding and controlling its behavior and impact during drug production and storage, rather than developing new therapeutic methods.
Package
![]()
![]()
![]()
![]()
Transportation
Small package(1g, 25g, 1Kg, 25Kg) can be shipped by Express. (DHL, FedEx, EMS, etc.)
Large package(100kg and more than100Kg) can be transported by Air or Sea.
All transportation is in accordance with the customer's needs.
Company Profile
![]()
![]()
![]()
![]()
![]()
![]()
Contact Person: admin
-
98+ White Powder Impurities(Standards) 6-Chloro-N-Methylpyridine-2-Carboxamide CAS 845306-04-9
-
98+ White Powder TICAGRELOR CAS 1129683-88-0 C14H14N4O3
-
98+ White Powder Impurities(Standards) Sorafenib Impurity 16 CAS 2206827-12-3
-
98+ White Powder Sorafenib Related Compound 8 NO. 1431697-81-2 CAS
-
98+ White Powder Sorafenib Impurity 6 CAS 1285533-84-7
-
Pure 98+ White Powder Sorafenib Impurity 3 CAS NO. 284670-98-0 C27H24N6O5