|
|
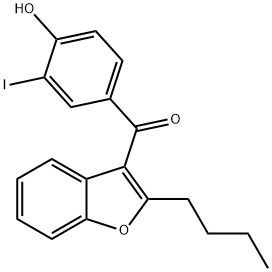
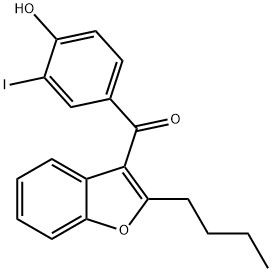
95+ White Powder Amiodarone EP Impurity F CAS 147030-50-0
|
Product Details:
Payment & Shipping Terms:
|
| Product Name: | Amiodarone EP Impurity F | CAS No: | 147030-50-0 |
|---|---|---|---|
| M.W: | 420.24 | MF: | C19H17IO3 |
| Appearance: | White Powder | Purity: | 95+ |
| EINECS No: | - | Storage: | 5-25°C |
| Highlight: | CAS 147030-50-0 Amiodarone EP Impurity F,White Powder Amiodarone EP Impurity F,95 Amiodarone EP Impurity F |
||
Product Description
White Powder Amiodarone EP Impurity F CAS 147030-50-0 95+
Name: Amiodarone EP Impurity F
CAS NO: 147030-50-0
M.W: 420.24
Appearance: White Powder
Purity: 95+
Synonyms: Amiodarone EP Impurity F
Application
Amiodarone EP Impurity F, as an impurity present in Amiodarone, does not have direct clinical applications or therapeutic functions. Amiodarone is a medication primarily used to treat arrhythmias, a condition where the heartbeat is irregular or abnormal. However, the impurity itself does not contribute to the therapeutic effects of the drug.
The primary application of Amiodarone EP Impurity F lies in drug quality control and safety assurance. Manufacturers and researchers monitor and control the levels of this impurity to ensure the purity and safety of Amiodarone. This involves using analytical techniques to detect and quantify the impurity accurately during the manufacturing process.
By controlling the presence of Amiodarone EP Impurity F, manufacturers can ensure that the final drug product meets the desired quality standards and poses minimal risks to patients. This is crucial in maintaining the efficacy and safety of Amiodarone as a treatment option for arrhythmias.
Package
![]()
![]()
![]()
![]()
Transportation
Small package(1g, 25g, 1Kg, 25Kg) can be shipped by Express. (DHL, FedEx, EMS, etc.)
Large package(100kg and more than100Kg) can be transported by Air or Sea.
All transportation is in accordance with the customer's needs.
Company Profile
![]()
![]()
![]()
![]()
![]()
![]()
Contact Person: admin
-
98+ White Powder Impurities(Standards) 6-Chloro-N-Methylpyridine-2-Carboxamide CAS 845306-04-9
-
98+ White Powder TICAGRELOR CAS 1129683-88-0 C14H14N4O3
-
98+ White Powder Impurities(Standards) Sorafenib Impurity 16 CAS 2206827-12-3
-
98+ White Powder Sorafenib Related Compound 8 NO. 1431697-81-2 CAS
-
98+ White Powder Sorafenib Impurity 6 CAS 1285533-84-7
-
Pure 98+ White Powder Sorafenib Impurity 3 CAS NO. 284670-98-0 C27H24N6O5