|
|
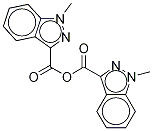
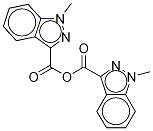
95+ White Powder Granisetron Impurity 1 CAS 1363173-34-5
|
Product Details:
Payment & Shipping Terms:
|
| Product Name: | Granisetron Impurity | CAS No: | 1363173-34-5 |
|---|---|---|---|
| M.W: | 334.33 | MF: | C18H14N4O3 |
| Appearance: | White Powder | Purity: | 95+ |
| EINECS No: | - | Storage: | 5-25°C |
| Highlight: | 95+ Granisetron Impurity,CAS 1363173-34-5 Granisetron Impurity,White Powder Granisetron Impurity |
||
Product Description
White Powder Granisetron Impurity 1 CAS 1363173-34-5 95+
Name: Granisetron Impurity 1
CAS NO: 1363173-34-5
M.W: 334.33
Appearance: White Powder
Purity: 95+
Synonyms: Granisetron Impurity 1
Application
Firstly, quality control is essential for guaranteeing the safety and effectiveness of drugs. Granisetron Impurity 1 serves as a key indicator in assessing the purity level of Granisetron. By accurately detecting and quantifying this impurity, manufacturers can assess whether the drug meets established quality standards, thus safeguarding patients' wellbeing.
Secondly, in the realm of drug research and development, understanding the nature and impact of impurities like Granisetron Impurity 1 is crucial for optimizing drug structures and synthesis processes. Studying this impurity can provide insights into the synthetic pathways and potential degradation mechanisms of Granisetron, enabling scientists to refine synthesis methods and enhance drug stability.
Lastly, production process monitoring is vital for identifying potential issues such as raw material contamination or incomplete reactions. By closely monitoring the levels of Granisetron Impurity 1, manufacturers can gain insights into the stability and reliability of their production processes. This, in turn, allows them to promptly adjust production parameters, ensuring consistent product quality and optimized production efficiency.
Package
![]()
![]()
![]()
![]()
Transportation
Small package(1g, 25g, 1Kg, 25Kg) can be shipped by Express. (DHL, FedEx, EMS, etc.)
Large package(100kg and more than100Kg) can be transported by Air or Sea.
All transportation is in accordance with the customer's needs.
Company Profile
![]()
![]()
![]()
![]()
![]()
![]()
Contact Person: admin
-
98+ White Powder Impurities(Standards) 6-Chloro-N-Methylpyridine-2-Carboxamide CAS 845306-04-9
-
98+ White Powder TICAGRELOR CAS 1129683-88-0 C14H14N4O3
-
98+ White Powder Impurities(Standards) Sorafenib Impurity 16 CAS 2206827-12-3
-
98+ White Powder Sorafenib Related Compound 8 NO. 1431697-81-2 CAS
-
98+ White Powder Sorafenib Impurity 6 CAS 1285533-84-7
-
Pure 98+ White Powder Sorafenib Impurity 3 CAS NO. 284670-98-0 C27H24N6O5