|
|
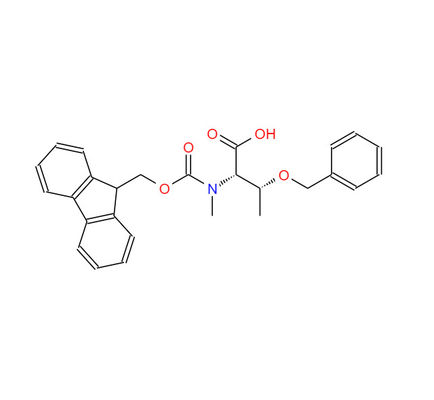
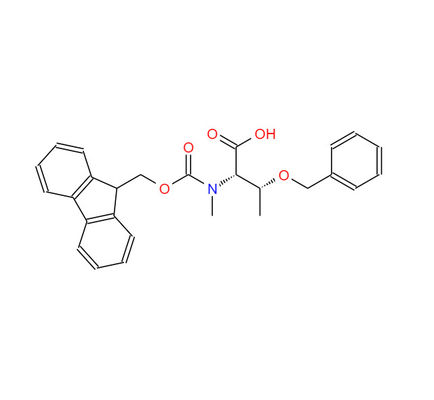
Fmoc-N-Me-Thr(bzl)-OH CAS 198561-81-8 Fmoc-N-methyl-O-benzyl-L-threonine
|
Product Details:
Payment & Shipping Terms:
|
| Product Name: | Fmoc-N-methyl-O-benzyl-L-threonine | CAS No: | 198561-81-8 |
|---|---|---|---|
| M.W: | 445.51 | Appearance: | White Powder |
| Purity: | 98+ | Storage: | Room Temperature5-25°C |
| Highlight: | Fmoc-N-Me-Thr(bzl)-OH CAS 198561-81-8,Fmoc-N-methyl-O-benzyl-L-threonine,L-threonine CAS 198561-81-8 |
||
Product Description
Fmoc-N-Me-Thr(bzl)-OH CAS 198561-81-8 Fmoc-N-methyl-O-benzyl-L-threonine
Name: Fmoc-N-Me-L-Thr(bzl)-OH
CAS NO: 198561-81-8
M.W: 445.51
Appearance: White Powder
Purity: 98+
Synonyms: N-(((9H-Fluoren-9-yl)methox)carbonyl)-O-benzyl-N-methyl-L-threonine; Fmoc-N-Me-Thr(bzl)-OH
Application
Peptide Libraries for Screening:
Fmoc-N-Me-Thr(bzl)-OH can be used in the synthesis of peptide libraries to explore how the N-methylation of threonine and the benzylation of the hydroxyl group impact stability, bioactivity, and binding affinity.
Peptide libraries can be screened to identify peptides with optimal stability, membrane penetration, or selectivity for target receptors, which can be important in drug discovery and therapeutic peptide development.
Therapeutic Peptide Design:
Fmoc-N-Me-Thr(bzl)-OH is useful in the synthesis of therapeutic peptides designed for improved stability, resistance to proteolysis, and target specificity. The N-methylation helps resist enzymatic cleavage, while the benzyl group protects the hydroxyl group during the synthesis process.
After synthesis, the removal of the benzyl group restores the hydroxyl functionality for any further modification or use in biological applications.
Package
![]()
![]()
![]()
![]()
Transportation
Small package(1g, 25g, 1Kg, 25Kg) can be shipped by Express. (DHL, FedEx, EMS, etc.)
Large package(100kg and more than100Kg) can be transported by Air or Sea.
All transportation is in accordance with the customer's needs.
Company Profile
![]()
![]()
![]()
![]()
![]()
![]()
Contact Person: admin
-
White Powder 98+ Fmoc-N-Me-Abu-OH CAS NO. 1310575-53-1
-
98+ White Powder Boc-N-Me-D-Phg-OH CAS 30925-12-3
-
HPLC 98+ White Powder Fmoc-N-Me-Trp-OH 112913-63-0 CAS
-
98+ Purity Fmoc-N-ME-LYS(Boc)-OH CAS NO. 197632-76-1
-
C6H13NO 98+ White Powder N-Me-D-Pro-Oh CAS No. 99494-01-6
-
C5H9NO4H2O N-Methyl-Amino AcidsN-Me-DL-Asp-OH CAS 303750-06-3